
Many different approaches are used in gene therapy, depending on the condition. Gene therapy can:
The definition of what constitutes a gene therapy has been evolving over the past 20 years. At this stage, there is broad consensus that there are 2 main types of gene therapy.
A functional gene is added directly into the target cell to replace the gene variant.
The added gene can:
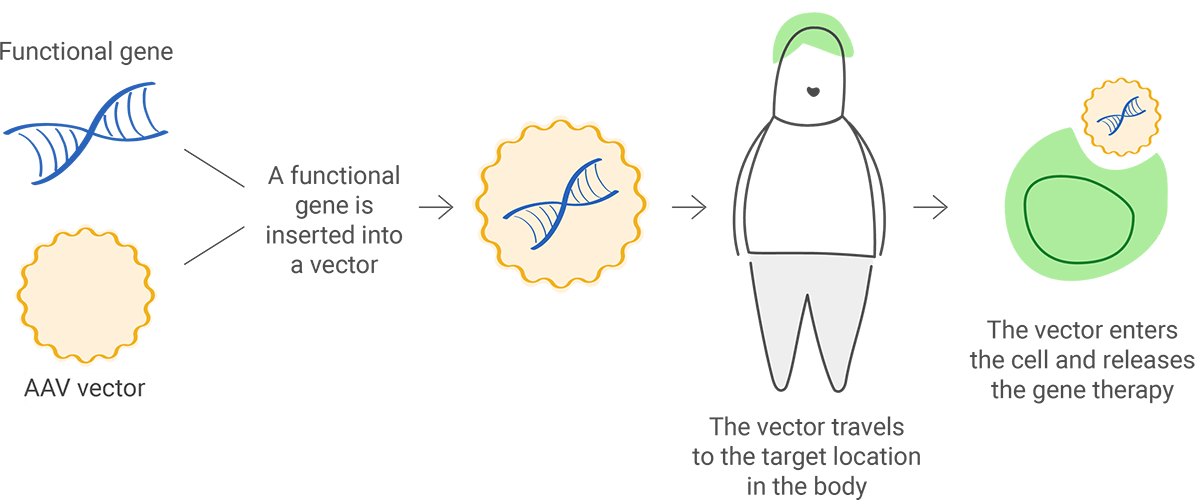
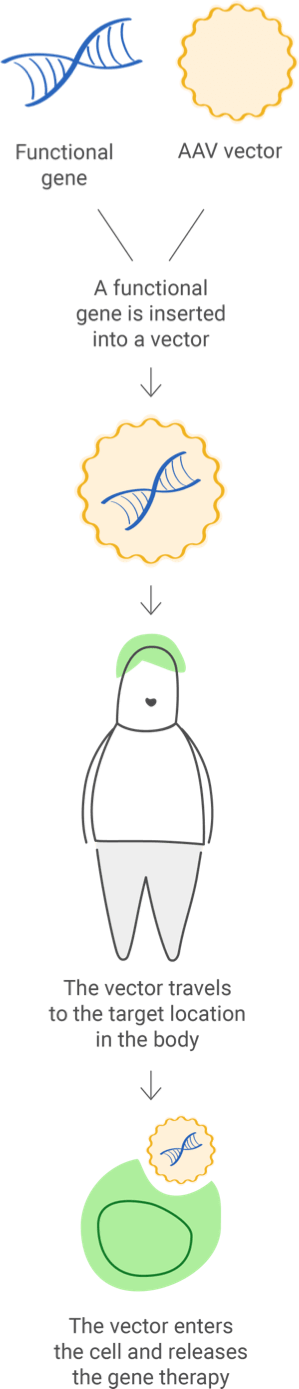
The functional gene can be inserted into a vector ("delivery vehicle") to carry DNA into the cell nucleus, but the newly inserted DNA does not change the foundational structure of genes.
Examples of therapy available
Adeno-associated viral vector delivery, gene replacement therapy
These technologies act like scissors, cutting the DNA at a specific spot. Then scientists can remove, add, or replace the DNA where it was cut.
Example of therapy available
CRISPR-Cas9 (clustered regularly interspersed short palindromic repeats and CRISPR-associated protein 9)
A sample of the patient's cells can be removed and exposed to the vector in a laboratory setting. The cells containing the vector are then returned to the patient.
The vector can be given intravenously (by IV) or injected directly into a specific tissue in the body, where it is taken up by individual cells.
Ex vivo gene therapy involves the genetic modification of cells outside of the patient's body.
One example of ex vivo therapy is CAR T cell therapy (or chimeric antigen receptor T cell therapy); it introduces a gene to a person's T cells, a type of immune cell. This gene provides instructions for making a protein that attaches to specific cells. The modified immune T cells can then recognize and attack disease-causing cells.
When in vivo gene therapy is used to introduce a new gene, it is packaged in a carrier (also called a vector) to reach a specific cell. Viruses are commonly used as vectors because they can be engineered to target specific cell types; but they are first modified so they can't cause disease in people.
Viral vectors can add and edit genes or modulate their expression. One type of viral vector that is widely used is adeno-associated viruses (AAVs), which are natural viruses that are different from adenoviruses. AAVs are smaller and less likely to cause an immune reaction than adenoviruses.
Scientists have been studying gene therapies since the 1970s, and clinical trials in patients have been conducted since the 1990s. As research progresses, genetic therapies hold promise to treat many diseases, but they are still a new approach and may carry risks. Potential risks could include certain types of cancer, allergic reactions, or damage to organs. Government regulatory bodies do a thorough assessment of the benefits and risks of a gene therapy when they review a treatment to permit its use as an investigational treatment in human clinical trials and/or when they finally approve a therapy for medical use.
Advances continue to improve gene therapy treatments and allow for a growing number of therapies to be approved for use in the US.
Before a gene therapy can be approved for use, it must be tested to assess whether the benefits of the therapy justify any risks.
Comprehensive federal regulations and guidelines—by the US Food and Drug Administration (FDA) and National Institutes of Health (NIH)—protect people participating in clinical trials. The FDA can reject or suspend clinical trials that are suspected to be unsafe.
Companies developing gene therapies and other treatments must demonstrate that they meet the FDA's standards for safety and quality before they can conduct a clinical trial in human patients.

First time gene therapy proposed as treatment for genetic disorders
First human test demonstrated safety of retroviral vector for gene therapy
First patient successfully treated with gene therapy for severe combined immunodeficiency
First human AAVs used in cystic fibrosis
In a clinical trial, a patient developed the first fatal response to an experimental gene therapy treatment for ornithine transcarbamylase deficiency
China approved world’s first commercial gene therapy for head and neck squamous cell carcinoma
First European Medicines Agency (EMA) approval of recombinant AAV1-LPL to treat lipoprotein lipase deficiency
FDA approval of first CAR T cell therapy to treat a form of acute lymphoblastic leukemia
FDA/European Union (EU) approvals of AAV2-RPE65 to treat inherited retinal dystrophy
FDA approval of AAV9-SMN1 for spinal muscular atrophy (SMA)
EU approval of AAV9-SMN1 for SMA
FDA approval of an AAV therapy for hemophilia and a lentiviral gene therapy for β-thalassemia
Gene therapy manufacturing is a multistep process requiring advanced technology and facilities to produce high-quality gene therapeutics.
Gene therapy manufacturing is a much more complex process than that of traditional medicines, such as small-molecule compounds that are derived from chemicals.
Developing clinical-grade viral vectors for use in gene therapy requires a cell-based, large-scale manufacturing system that supports living cells so that the functional gene can be inserted into a viral vector.
Strict controls to keep sterile conditions
Safety & efficacy
Cells need to be maintained in controlled conditions
A single gene therapy treatment may require hundreds of thousands or millions of cells depending on the target in the body
Significant progress has been made in manufacturing safe, efficient, and economical delivery systems for therapeutic genetic material and viral vectors. Researchers continue to develop novel approaches to improve the capacity and capabilities of the technology.
This is not a comprehensive list of resources, and it is provided for reference only.